Pharmacogenomics: reading genes to tailor prescriptions for individuals

The old medical advice to “start low and go slow” reflects a fundamental challenge in healthcare: the same drug at the same dose can heal one patient while harming another. For decades, physicians have prescribed medications based on population averages, essentially conducting a trial-and-error experiment with each patient. Today, pharmacogenomics is rewriting this script by revealing how our genes influence drug response, transforming medication prescribing from guesswork to precision.
The drug-gene connection
Pharmacogenomics examines how genetic variations affect an individual’s response to medications, determining whether a drug will be effective, ineffective, or even dangerous for a particular person. Unlike traditional medicine’s one-size-fits-all approach, this field recognises that genetic differences can dramatically alter how our bodies metabolise and respond to drugs. Mostly, this variability arises from differences in drug-metabolising enzymes, particularly the cytochrome P450 (CYP) family, which processes approximately 75% of commonly-prescribed medications.
Genetic variations in these enzymes create different metaboliser phenotypes. A “poor metaboliser” with low functional enzyme activity may accumulate toxic drug levels from standard doses, while an “ultrarapid metaboliser” with enhanced enzyme activity may receive no therapeutic benefit. These aren’t rare genetic quirks. Studies show that approximately 90% of people carry at least one actionable pharmacogenetic variant. The clinical implications are profound: genetic factors contribute significantly to adverse drug reactions (ADRs), which rank among the leading causes of hospitalisation and death in developed nations.
Real-world impact
The translation of pharmacogenomics from the laboratory to the clinic, has begun to deliver measurable gains across diverse areas of medicine. A clear example is Warfarin, a widely prescribed blood thinner with a notoriously narrow therapeutic window. Genetic variants in the CYP2C9 and VKORC1 genes account for approximately 50% of the variation in Warfarin dose requirements. Patients carrying certain variants may need only one-third of the standard dose to achieve therapeutic effect. Pharmacogenomic-guided dosing algorithms that incorporate these genetic markers have demonstrated improving outcomes, reducing adverse bleeding risks and helping patients reach therapeutic levels faster than traditional trial and error dosing methods.
In cardiovascular medicine, Clopidogrel, a cornerstone antiplatelet drug used after heart attacks and stenting, provides another compelling example. The drug requires activation by the CYP2C19 enzyme. Patients with loss-of-function CYP2C19 variants, particularly CYP2C19*2 (found in 25-30% of the population), exhibit reduced drug activation and significantly higher risks of cardiovascular events including stent thrombosis. Reflecting this evidence, the 2022 Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines now recommend alternative antiplatelet agents for CYP2C19 poor metabolizers undergoing percutaneous coronary intervention.
Beyond cardiology, psychiatry has also emerged as a fertile ground for pharmacogenomic implementation. Many antidepressants and antipsychotics are extensively metabolised by CYP2D6 and CYP2C19. The genetic variants in these enzymes can profoundly influence drug levels and side effects. Pre-emptive pharmacogenomic testing in psychiatric patients has demonstrated reduced adverse drug reactions, improved symptom control, and decreased healthcare costs. Oncology, too is advancing rapidly under the pharmacogenomics lens. Testing for variants in the DPYD gene before administering chemotherapy drugs like 5-fluorouracil can prevent severe, life-threatening toxicity.
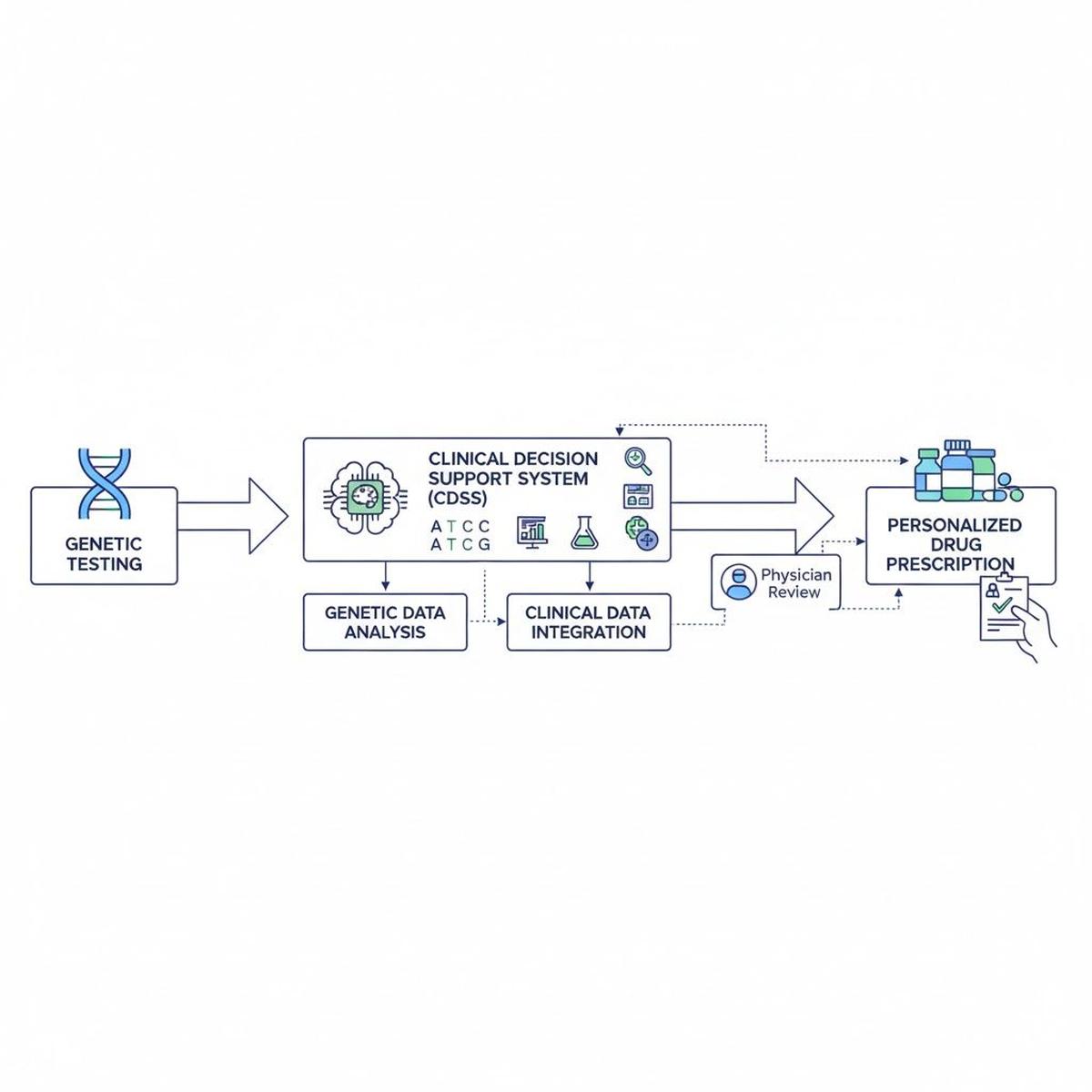
Flowchart illustrates the pathway from genetic testing through clinical decision support systems to tailored drug prescriptions, emphasizing the integration of genomics into healthcare workflows.
The economic equation
The economic case for pharmacogenomics lies in balancing the upfront cost of genetic testing with long-term savings achieved through fewer adverse events and improved therapeutic outcomes. Over the past decade, genetic testing costs have fallen dramatically i.e. from thousands of dollars to roughly $200-500 for comprehensive panels today. Yet the true value of pharmacogenomics extends far beyond the price. Economic analyses consistently show that genetic-guided prescription is cost effective for several drug-gene pairs, particularly in chronic disease where patients require long-term medication management.
To assess this value, experts use a framework that considers several factors, such as severity and cost of side-effects, the frequency of genetic variants in the population, the availability of alternative drugs, and range of medications influenced by variations. For example, screening for HLA-B*57:01 before prescribing Abacavir, or for HLA-B*15:02 before Carbamazepine, helps in the prevention of potentially adverse reactions such as fatal Stevens-Johnson syndrome, making these test unequivocally cost-effective. The economic benefits compound when considering pre-emptive or panel-based testing strategies, where a single genetic test can provide guidance for dozens of medications throughout a patient’s lifetime. Such approaches transform pharmacogenomics from a one-time diagnostic expense into a long-term investment in safer and more efficient healthcare.
However, the cost-effectiveness of pharmacogenomic testing varies significantly across different clinical settings. In acute, short-term conditions with limited treatment duration, routine testing may not be justified. But in chronic diseases that involve multiple medication trials such as depression, cardiovascular disorders, or chronic pain; the economic case is much stronger. Increasingly, healthcare systems recognise that preventing even one serious adverse drug reaction often offsets the cost of testing multiple patients.
Implementation challenges
Despite compelling scientific evidence, pharmacogenomics still faces substantial barriers towards widespread clinical adoption. A 2023 scoping review identified knowledge gaps among healthcare providers as the primary obstacle. Most physicians and pharmacists receive minimal pharmacogenomic education in training, leaving them ill-equipped to order, interpret, and apply genetic test results. Beyond education, infrastructure limitations compound this challenge; many electronic health record systems lack adequate decision-support tools to integrate pharmacogenomic data into prescribing workflows. Without such digital assistance, clinicians often struggle to act on genetic information in real time.
Reimbursement uncertainty creates additional hesitation. While some health systems and insurers cover pharmacogenomic testing for specific indications, coverage remains inconsistent across payers and jurisdictions. Meanwhile, regulatory pathways continue to evolve: over 100 Food and Drug Administration (FDA) drug labels now contain pharmacogenomic information, yet the clinical implications of these labels vary as some offer actionable recommendations while others are merely informative statements.
Finally, cultural and institutional resistance should not be underestimated. Changing prescribing practices requires not just evidence but also trusted implementation frameworks, local champions, and administrative support. Encouragingly, where implemented, these programmes demonstrate that systematic approaches addressing education, infrastructure, and workflow integration can successfully overcome these barriers.
The path forward
The future of pharmacogenomics lies in pre-emptive testing strategies, where genetic information is obtained before a medication is needed and remains available throughout a patient’s healthcare journey. Several health systems now offer pre-emptive pharmacogenomic panels to high-risk populations, integrating results into electronic health records to trigger alerts when relevant medications are prescribed. As whole-genome sequencing becomes increasingly affordable, pharmacogenomic information may become a routine component of preventive medicine.
Ultimately, pharmacogenomics represents a fundamental shift in how we think about drug therapy from treating populations to treating individuals, from reactive care to proactive prevention, from trial-and-error to precision medication. The prescription, quite literally, is written in our genes: we are now learning how to read it.
(Dr. Renu Yadav is senior demonstrator, PGIMER, Chandigarh. gomailtorenu@gmail.com)
Published – November 21, 2025 06:00 am IST